
#ATOMIC RADIUS PERIODIC TREND SERIES#
An isoelectronic series is useful in understanding the effects of gained or loss electrons on atom size. Cl -, Ca 2 +, Ar all have 18 electrons therefore, they are isoelectronic (F - has 10 electrons).This will cause the electrons push each other away and spread out, causing the atom to become larger. When this occurs there are more electron-electron repulsions and there is a lower net nuclear attraction per electron. Anions are formed when an electron is gained. So, the newly formed ion becomes a more condensed version of its neutral atom. When this occurs there are less electron-electron repulsions and there is a greater net nuclear attraction per electron. C & D: Cations are formed when an electron is lost.However, for less symmetrical and more polar lattices such as those with C n, C nh, and C nv symmetries, significant changes in the electron density can occur, causing deviations from spherical shape these deviations make ionic radii more difficult to measure.


For instance, lattices with O h and T d symmetries are considered to have high symmetry thus the electron densities of the component ions occupy relatively-spherical regions and ionic radii can be measured fairly accurately. The point group symmetry of a lattice determines whether or not the ionic radii in that lattice can be accurately measured (Johnson 1973). For a given ion, the ionic radius increases with increasing coordination number and is larger in a high-spin state than in a low-spin state.Īccording to group theory, the idea of ionic radii as a measurement of spherical shapes only applies to ions that form highly-symmetric crystal lattices like Na + and Cl. Ionic radius is not a permanent trait of an ion, but changes depending on coordination number, spin state, and other variables (Shannon 1976). After comparing many compounds, chemist Linus Pauling assign a radius of 140 pm to O 2- and use this as a reference point to determine the sizes of other Ionic Radii (Jensen 2010). However, it is to consistently and accurately determine the proportions of the ionic bonds. The ionic radius of an atom is measured by calculating its spatial proportions in an ionic bond with another ion within a crystal lattice.

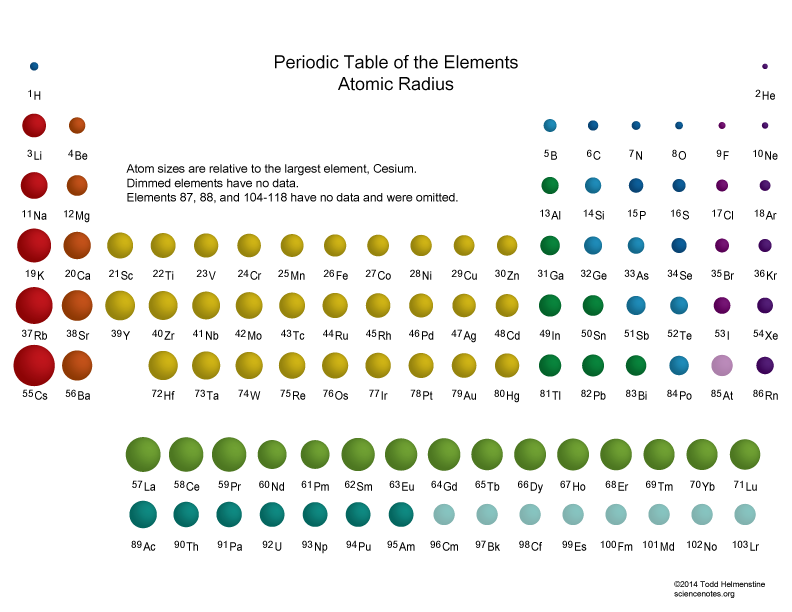
Measurement and Factors Affecting Ionic Radii This is because it will increase with decreasing atomic radius, since a smaller atom will have a greater pull on electrons from the positively charged nucleus.\( \newcommand\) This pattern is the same as ionization energy, increasing as you move up and to the right on the periodic table. For the same reason, ionization energy will also increase moving left to right across a period, since atomic radius also decreases in this pattern.Įlectronegativity: Electronegativity is the ability of an element to attract electrons. This will thus increase moving up on the periodic table, since it will require more energy to take an electron from an inner shell that is closer to the positively charged nucleus. Ionization Energy: Ionization energy is the energy needed to remove an electron from an atom. This is because you are adding more electrons into the same shell, so there is more negative charge which has an increasingly stronger attraction to the positively charged nucleus, pulling the electron shells in closer and thus decreasing the overall atom size. Going across the groups, however, the atomic radius decreases from left to right. Atomic Radius: Going down the periodic table, each period has higher atomic radius because each period represents an additional electron shell being added, which means the atoms must grow larger.


 0 kommentar(er)
0 kommentar(er)
